Dispersants vs. wetting agents: what is the difference?
Dispersants vs. wetting agents: what is the difference?
We often hear wetting and dispersing agents mentioned together, almost as one additive category. However, wetting agents and dispersants do not play the same role in the formulation.
Dispersion of pigments in paint happens in 3 steps:
- Wetting agents ease the wetting of solid particles,
- Dispersing agents ensures the stability of the dispersion over time, and
- Mechanical forces ensure the separation of particles
Wetting agents are not always needed – they are majorly used in case you work with hydrophobic pigments in a water-based system. Determine whether you need wetting agents in your formulation.
In this guide, we will focus on dispersants only. We may use dispersants and dispersing agents randomly to speak of this ingredient category.
What is a dispersing agent or dispersant?
What is a dispersing agent or dispersant?
A dispersing agent by definition is a paint ingredient which is used to avoid the flocculation of pigments and fillers. In other words, dispersants promote and stabilize the suspension of solid particles in the paint medium.
 All dispersing agents have two parts:
All dispersing agents have two parts:
-
Anchoring groups
-
Soluble tails
The choice of your dispersant will greatly influence the quality of your dispersion. They improve:
How does a dispersant work?
How does a dispersant work?
Dispersants function by reducing the attraction between solid particles like pigments, fillers, or additives in any dispersion. This prevents the solid particles from agglomerating or settling out.
Generally, when solid particles are added to any liquid medium, they tend to attract each other due to intramolecular forces. Dispersants function is to initially separate these particles from each other and continuously avoid further agglomeration.
First, anchoring groups adsorb to the pigments and fillers surface. They form strong physical bonds with chemical groups that are present on the surface of the particles, the so-called anchor sites.
Second, soluble tails create a layer around the solid particles preventing dispersed particles to glue together again and ensuring colloidal stability. These repulsive forces working against the natural attraction of particles are of two kinds:
- Electrostatic repulsion: The surface of particles is equally charged and they repel each other.
- Steric repulsion: The tails form a layer around the solid particles and prevent particles to collide with each other.
Here is a short video showing dispersant mode of action:

The mode of action is quite simple. The selection of the right dispersant is not. There are more than 2600 dispersing agents available on the market today!
Why are there so many different commercial dispersants to choose from?
Why are there so many different commercial dispersants to choose from?
If there is no affinity between the anchor groups of the dispersant and the anchor site of the solid particles, bonding fails, and the dispersant can’t play its role in the formulation.
Chemicals functional groups (anchor sites) at the surface of pigments and fillers vary a lot. Some are surface-treated, and others are not. So that proper anchoring can take place, dispersing agents also come with a great variety of functional groups. To name a few, you will find grades with amine, amide, sulfonate, or phosphate anchoring groups.
On the other hand, the dispersant tail must be soluble in the paint medium and compatible with the binder. Again, depending on the binder and solvent characteristics, formulators need different solutions.
This is the reason why the dispersant offering is so wide.
We are partnering with more than 10 suppliers, covering the full chemistry spectrum, in order to help you test materials in your lab. In case, you feel lost in front of the plethoric dispersant offering, ask our material specialists for help!
What are the key selection criteria for dispersing agents?
What are the key selection criteria for dispersing agents?
Choose the best fitted stabilization method
The first question to answer when selecting your dispersing agent is whether you need electrostatic or steric repulsion. The answer is highly dependent on the system you formulate: Is it solvent-based? Water-based?
If your paint medium is apolar (solvent-based paints), the answer is simple. Electrostatic stabilization is impossible. Steric stabilization is the only way.
With a polar medium (water-based and some solvent-based formula),
- Electrostatic stabilization is the default way. This is for example the kind of stabilization mechanism that you would find in wall paints.
- In case of more complex systems, steric stabilization can prove useful.
- Nowadays, many modern dispersants are offering electrosteric stabilization. They provide both electrostatic and steric stabilization in a single molecule.
Anchor sites at the surface of your pigments and fillers
As your dispersant molecules need to bond with the solid particle, it is important to identify the anchoring opportunities you have with your pigments and fillers:
- Are you trying to stabilize organic pigments? Inorganic?
- Are you working with naked or surface-treated particles?
It is important to know so you can choose a molecule that can adsorb on the solid particle. Hydrogen bonding is the most frequent mechanism involved. You may hear people speak of acid/base or donor/acceptor interactions.
Good binder compatibility
The non-adsorbing moieties of the dispersing agent should demonstrate excellent compatibility with the binder system. This is essential for optimal stability in the liquid phase as well as best film performance. Any incompatibility may result in pigment flocculation in the wet paint, even during film formation.
Assistance to select the best fitted dispersants
At anytime, if you struggle defining which stabilization method would be best, and then match chemical groups between your pigments or fillers and dispersing agents, remember that our team of dispersant specialists is happy to help you. Simply ask!

Different Types of Dispersing and Their Chemistries
Different Types of Dispersing and Their Chemistries
There are many dispersing agents chemistries today in the coatings market, and choosing the right one for your system is tricky. The chemistry of your dispersing agent plays an important role in the performance and success of your final coating. The optimal dispersing agent chemistry can impact the stability, quality, and performance of your dispersion or coating.
Various dispersing agent classes can be classified as below:
- Conventional Dispersing Agents (High Mw, Low Mw)
- Polymeric Dispersing Agents
- Ionic and Non-ionic Dispersing Agents
Let’s find out more about the different chemistry types of dispersing agents.
Conventional Dispersing Agents
Mainly low molecular weight, they are based on polyesters, polyamides, polyglycols and fatty acid chemistry (FAME). They have general characteristics as listed below:
- Surfactant effect, reduction of solid / liquid interface surface tension
- Anchoring groups adsorbed at the pigment surface
- Good compatibility with the media
- MW = 500 ~ 2,000 g/mol
Other key features of this type of dispersants include:
- Excellent wetting power
- Grinding / dispersing time reduction
- Anti-sedimentation
- Effective against flooding and floating
- Action mode: Mainly electrostatic, few steric hindrance
- Recommended for inorganic materials and waterborne systems suitable for organic pigments
High molecular mass dispersants (Mw approx. 5000-30,000 g/mol) – They are most widely used in industrial paints. As compared to low molecular mass dispersants, they typically provide:
- Superior performances,
- Workability,
- Best coloristic properties,
- Gloss,
- Film transparency,
- Film integrity,
- Low risk of being extracted from the dried film, and
- Minimal side effects.
High Versus Low Molecular Mass -based Dispersants
High molecular mass-based additives tend to be more system specific as compared to low molecular mass dispersants and a careful selection and evaluation procedure is required.
Oligomers of medium high molecular mass (Mw around 1000-2000 g/mol) typically show widest range of compatibility and superior (fast) pigment wetting properties in comparison to high molecular mass products, whereas still outperforming low molecular mass products in dispersion stability and film consistency properties.
With few exceptions, monomolecular surfactant-based additives are less commonly used as dispersing agent. This is because, this group typically is inferior in contributing to dispersion stability and has high risk on effecting film properties, such as:
If you struggling at achieving the desired characteristics - hardness, resistance to water and solvents or adhesion even you have the right chemistry, then it may come from an overuse of dispersants. Take this quick tutorial to save on troubleshooting and understand if you are using optimum level of dispersants (and hence, lower formulation costs).
Polymeric Dispersants
Main dispersing agents that are polymeric include polyacrylates, polyester, polyether or polyurethane-based systems. The classification of polymeric types dispersants is based on their:
- Anchoring mechanism
- Chemical structure (polyacrylic, polyurethane, copolymer…), and
- Molecular weight
This type is also influenced by the polymer design (linear, branched, star designed) and the polymerization process (controlled polymerization process types offer high performances products but are also more expensive). Their key characteristics include:
- Polymeric type: many anchoring groups
- Large choice of chemistry
- Large choice of polymer design and molecular weight
- Mw = 5,000 ~ 50,000 g/mol
Further, polymeric dispersing agents offer several benefits as listed below.
- Excellent wetting power
- Grinding / dispersing time reduction
- Very effective for the long term stabilization
- Action mode: steric hindrance
- Polyvalent family (waterborne, solventborne, organic or inorganic material)
Polyacrylic Acid Based / Polyacrylates - Polyacrylic acid-based dispersants are usually lower in molecular weight (and also in cost) in comparison with the other structures. They are particularly recommended in water-based paints to increase the pigment load of inorganic material. Very nice cost effective product. Ammonium and sodium salt are typical products for latex paints - higher in molecular weights, they can offer a better compatibility.
Polyurethanes - They are excellent for the millbase viscosity reduction. As a consequence, PU dispersants enhance the pigment load and reduce the dispersing time. The flexibility of this structure (backbone, branched chains, anchoring groups) allows the design of various structures for many solventborne and solvent-free systems.
View the Full Range of Polymeric Dispersing Available today »
Controlled Polymerization Technology (CPT)/ Living Chain Growth
This polymerization process allows the manufacturer to make very fine adjustment on the polymer chain, which is not the case with the classical step-growth process (condensation polymerization is a random process).
Dispersing agent polymerized with this process are very similar batch to batch, which is not the case of classical condensation where the molecular weight can vary significantly from one batch to the other. Very effective but more expensive products.
The table below compares the properties of conventional and polymeric dispersing agents.
| Property |
Conventional |
Polymeric |
| System |
Waterborne |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
| Solventborne |
⭐ ⭐ |
⭐ ⭐ ⭐ |
| Pigment |
Organic |
⭐ ⭐ |
⭐ ⭐ ⭐ |
| Mineral |
⭐ ⭐ |
⭐ ⭐ ⭐ |
| Electrostatic Stabilization |
High |
Low |
| Steric Hindrance Stabilization |
Low |
High |
| Pigment load |
Low - Medium |
High |
| Final pigment paste quality |
Low - Medium |
High – Very high |
| Versatility |
Medium |
High |
| Price |
Low - Medium |
High – Very high |

Ionic and Non-ionic Dispersants
For use in water-based paints or coatings, anionic charged and non-ionic dispersing agents can be considered. Excellent wetting and dispersion performance in mill-bases for dispersion paints can be achieved using a combination of sodium- or ammonium-polycarboxylate and polymeric non-ionic surfactant additive. A main non-ionic additive is alkyl phenol ethoxylate (APE) and more precisely nonyl phenol ethoxylate, NP 10 (ethylene glycol chain of 10 units). Due to toxicity concerns, NP 10 is being replaced now with an APE free non-ionic, possessing same HLB-value.
HLB stands for hydrophilic lipophilic balance and is used as indicative value for comparing nonionic surfactants from different hydrophobic nature. The HLB value can be calculated from the percentage hydrophilic components in a molecule, divided by 5.
 Effect of the Dispersing Agent on Color Development PBk in a Stoving Enamel.
Effect of the Dispersing Agent on Color Development PBk in a Stoving Enamel.
High molecular Mass Dispersant (L), Right Low Molecular Mass Dispersant (R)
Related to the high degree of ionic dissociation in water, applying combinations of anionic and cationic dispersants in aqueous systems should be avoided; reaction between the anionic and cationic products may result in insolubility and changed surface activity.
Cationic, amine functional dispersants are successfully used in solvent-borne systems, for instance to support the wetting and dispersing process. Due to the low degree of dissociation the effect of the electronic charge is less evident in a-polar systems.
Select the Right Dispersing Agent for Your Formulation
Select the Right Dispersing Agent for Your Formulation
Selecting the best wetting and dispersing agent for a system may look complicated first, but many clues can orientate our choice. Here is an overview of most commonly-used chemistries depending on the end-use application.
Dispersant Performance According to Your End-use Application
| End-use Application |
Conventional |
Polymeric |
| Polyacrylic acid |
Polyurethane |
Polyacrylates |
CPT |
| Architectural (Interior) |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ |
⭐ |
⭐ |
| Architectural (Exterior) |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ |
⭐ |
⭐ |
| Automotive |
⭐ ⭐ |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
| Can / Coil |
⭐ ⭐ |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ |
| General Industry |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ |
| Printing |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ |
| Wood / Flooring |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ |
| Resin Containing Concentrates |
⭐ ⭐ |
⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ |
| Resin Free Concentrates |
⭐ |
⭐ |
⭐ ⭐ ⭐ |
⭐ ⭐ ⭐ |
⭐ |
| Universal Pigment Concentrate |
⭐ ⭐ ⭐ |
⭐ |
⭐ ⭐ |
⭐ ⭐ |
⭐ |
It can be concluded that: selecting the right dispersing agent is a compromised based on many parameters.
-
First, the system itself (waterborne or solvent borne)
-
Then the pigment (organic, mineral, fine, rough, transparent…)
-
And finally, the end-use application
In some formulation, changing the dispersing agent is a really positive choice, enhancing the paint quality. Products from the CPT offer excellent results, but is the cost reasonable in the considered formulation? The right choice will be based on these test results of course, but also on the specification to reach, not only in terms of paint quality, but also economically.
If you work on tight schedule, it may be faster to speak with a dispersant expert and get a specific recommendation for your system.
Role of Dispersants in Preventing Sedimentation
Role of Dispersants in Preventing Sedimentation
In general, solid particles in a liquid are pulled down because of gravitational force. The cause for this phenomenon is that most pigments and fillers have a density that is higher than the density of the liquid that surrounds the particles. The process of particles sinking in a liquid, called sedimentation, can give problems during storage. In the extreme case, particles can cluster together on the bottom of the can. This phenomenon, resulting in the formation of hard sediment, is called settling.
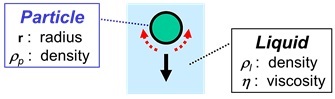 Sedimentation of a Solid Particle in a Liquid
Sedimentation of a Solid Particle in a Liquid
Several properties govern the speed of sedimentation:
- An important factor, governing how fast a particle will go down, is the density of the particle or, to be more precise, the difference in the density of the particle and the density of the surrounding liquid (ρp - ρl).
- The second factor is size: big particles sink faster than small particles.
- The third factor is the viscosity of the surrounding liquid. Liquid has to make place when a particle goes down: liquid must flow around the particle to fill-up the space where the particle comes from. This flow of liquid is easier when the viscosity of the liquid is lower. A low viscosity of the liquid will, therefore, result in faster sedimentation.
Related Read: Get clarity around the core fundamentals for efficient management of the rheological profile of your product
Sedimentation is mainly a problem that occurs during storage of the system. During storage, the system stands still and the main force acting on the system is the gravitational force. Sinking of particles can be prevented by arranging a physical network in the system.
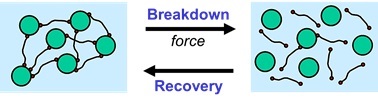 Reversible Physical Network Preventing Sedimentation
Reversible Physical Network Preventing Sedimentation
Because of the physical network, the particles behave as if they are frozen-in during storage when the applied force is low. During storage, the liquid paint or ink can be considered as a solid system when the physical network is strong enough. As soon as enough force is applied, for example by mixing, brushing, pumping or spraying, the physical network breaks down, and the system behaves as a liquid.
A physical network can be built-up in a liquid system by using rheology additives, often referred to as associative thickeners. The materials are called ‘associative’ because they attach themselves onto fellow molecules or they adsorb partly at the surface of particles that are present in the system.
The physical network, obtained by using rheology additives, has to be strong enough to resist the gravitational force during storage. On the other hand, the network must be weak enough to be broken down as soon as enough force is applied.
How dispersants work with different pigments?
How dispersants work with different pigments?
Pigments are usually the most expensive raw material in paint systems and can only show their full color strength if optimally dispersed. High performance dispersants can provide the required color quality with the minimum amount of pigment and, thus, help to minimize raw material costs.
Easily Wetted Pigments Vs Difficult to Disperse Pigments
The necessity to contribute to wetting depends on the pigment liquid phase characteristics. Easy wetted pigments, like Titanium Dioxide (TiO2) in water, do not require additional wetting support, so emphasize on the contribution of the dispersant is very much on stabilization effect.
The main group of dispersants as used in white water-based dispersion paints is sodium-polycarboxylate. Indeed, this dispersant provides excellent electrostatic stabilization, but provides only limited wetting activity.
However, another important pigment – Carbon Black – is difficult to disperse and stabilize, primarily due to their notoriously low surface charge and poor wetting characteristics. Carbon black pigments provide excellent color and hiding power and can ultimately improve coating performance.
But at the same time, carbon black is generally considered to be the most time consuming and difficult pigment to disperse. This is especially true for waterborne systems because water is very polar, has high surface tension, and there is little interaction between the binder and the pigment. These properties require the use of a highly efficient wetting and dispersing agent.
Organic pigments are high in tint strength and brightness, but they are very difficult to disperse and stabilize because of small particle size. The small particle size causes following issues inhibiting wetting and dispersant adsorption :
- Increased flocculation
- Non-uniform surface structure
- Low surface energy
Dispersants as offered for organic pigments in water demonstrate strong wetting support, as well as stabilization activity. A wide range of products is offered, however, having in common of offering surfactant (reduction interfacial tension pigment and liquid phase) as well as strong stabilization properties.
Can science-based approach help you go faster in selection process? One possible approach to quickly predict the most compatible dispersant/pigment pairing is by using Hansen Solubility Parameters. Read this case study utilizing 2 carbon black grades (Raven 5000 Ultra II and Raven 5100 Ultra) and 2 dispersants (Tego® Dispers 761 W and CLiQSPERSE® 149), where compatibility predictions were made and validated via practical experiments.
 CASE STUDY CASE STUDY
Optimizing Coating Performance via Predictive Compatibility Parameters of Carbon Blacks and Dispersants
» READ CASE STUDY |
Now that you have learned about all the selecting criteria, we now focus on the quick tips which are useful for you to test the efficiency of your selected chemistry.
How can you evaluate the efficiency of Wetting & Dispersing Agent?
How can you evaluate the efficiency of Wetting & Dispersing Agent?
The wetting & dispersing agent has a significant influence on the paint properties. It has a direct impact on the particle size, and then, its efficiency can be evaluated by checking the right parameters.
To complete its validation, the wetting & dispersing agent must follow a serial of laboratory tests as discussed below.
-
Compatibility with the System - Mix the wetting & dispersing agent with the system, without pigments. It should be perfectly compatible with the other formulation component. If not, try adjusting the pH or the polarity.
- Pigment Shock - After the dispersion, make a simple poor-out of a small amount of paint diluted (10-20% in solvent or water). Pigment shock results of a poor pigment stabilization.
- Draw down - Make a simple draw down and check the quality of the application - Color strength, transparency, gloss, general aspect. Incompatible wetting and dispersing agent can lead to many defects like seeding.
- Rub out (for color mix, or pigment concentrates in a base paint) - In order to check the flooding, a simple rub out test can be done. After short drying time when the film is nearly dry, with the finger rub a part of the paint surface. The color should be the same as the unrubbed part.
- Storage Stability - Paint samples are stored at cold temperature (-5°C to 5°C) and high temperature (40°C to 60°C) for one or two weeks and the previous tests are realized, then the results are compared with the original ones and the sample stayed on the shelves.
A perfect product should not show any significant variation regarding the storage conditions.
So, what are you waiting for? Let’s start formulating!
Our Coatings Selector features a full range of dispersing agents available today for all types of coating and ink formulations (aqueous, solvent-based, high-solid or 100% solids systems and for pigment concentrates). You can check tech profile for each product, ask for samples or discuss your case with producer’s tech staff.
We would like to acknowledge our experts Jochum Beetsma, Johan Bieleman & Vincent Makala for providing technical information needed to develop this page.